Do All Liquids Expand at the Same Rate? How Do You Know?
Learning Objectives
By the finish of this section, yous volition be able to:
- Define and describe thermal expansion.
- Summate the linear expansion of an object given its initial length, alter in temperature, and coefficient of linear expansion.
- Calculate the volume expansion of an object given its initial volume, change in temperature, and coefficient of volume expansion.
- Summate thermal stress on an object given its original book, temperature change, volume change, and bulk modulus.

Figure 1. Thermal expansion joints like these in the Auckland Harbour Bridge in New Zealand allow bridges to change length without buckling. (credit: Ingolfson, Wikimedia Commons)
The expansion of alcohol in a thermometer is one of many commonly encountered examples of thermal expansion, the change in size or volume of a given mass with temperature. Hot air rises because its volume increases, which causes the hot air's density to exist smaller than the density of surrounding air, causing a buoyant (upward) force on the hot air. The aforementioned happens in all liquids and gases, driving natural oestrus transfer upwardly in homes, oceans, and conditions systems. Solids also undergo thermal expansion. Railroad tracks and bridges, for example, take expansion joints to allow them to freely expand and contract with temperature changes.
What are the basic backdrop of thermal expansion? First, thermal expansion is clearly related to temperature change. The greater the temperature change, the more a bimetallic strip volition bend. 2d, it depends on the material. In a thermometer, for instance, the expansion of alcohol is much greater than the expansion of the glass containing information technology.
What is the underlying crusade of thermal expansion? As is discussed in Kinetic Theory: Atomic and Molecular Explanation of Pressure level and Temperature, an increase in temperature implies an increase in the kinetic energy of the private atoms. In a solid, different in a gas, the atoms or molecules are closely packed together, but their kinetic energy (in the form of pocket-sized, rapid vibrations) pushes neighboring atoms or molecules apart from each other. This neighbor-to-neighbour pushing results in a slightly greater distance, on average, between neighbors, and adds up to a larger size for the whole body. For most substances under ordinary conditions, in that location is no preferred direction, and an increase in temperature will increase the solid's size past a certain fraction in each dimension.
Linear Thermal Expansion—Thermal Expansion in One Dimension
The modify in length Δ50 is proportional to length L. The dependence of thermal expansion on temperature, substance, and length is summarized in the equation ΔL=αLΔT,where ΔL is the alter in length L, ΔT is the change in temperature, and α is the coefficient of linear expansion, which varies slightly with temperature.
Table 1 lists representative values of the coefficient of linear expansion, which may take units of 1/ºC or 1/Grand. Because the size of a kelvin and a degree Celsius are the same, both α and ΔT can be expressed in units of kelvins or degrees Celsius. The equation ΔL=αLΔT is accurate for minor changes in temperature and can be used for large changes in temperature if an average value of α is used.
| Table 1. Thermal Expansion Coefficients at 20ºC[1] | ||
|---|---|---|
| Cloth | Coefficient of linear expansion α(1/ºC) | Coefficient of volume expansion β(ane/ºC) |
| Solids | ||
| Aluminum | 25 × 10 – half-dozen | 75 × 10 – 6 |
| Brass | 19 × 10 – six | 56 × x – 6 |
| Copper | 17 × 10 – 6 | 51 × 10 – half-dozen |
| Golden | 14 × 10 – vi | 42 × 10 – half dozen |
| Fe or Steel | 12 × 10 – 6 | 35 × ten – six |
| Invar (Nickel-iron alloy) | 0.9 × 10 – 6 | 2.7 × 10 – 6 |
| Pb | 29 × 10 – half dozen | 87 × 10 – 6 |
| Silver | 18 × 10 – 6 | 54 × 10 – vi |
| Glass (ordinary) | 9 × 10 – vi | 27 × 10 – 6 |
| Glass (Pyrex®) | iii × 10 – six | ix × 10 – six |
| Quartz | 0.4 × 10 – 6 | 1 × 10 – six |
| Concrete, Brick | ~12 × x – 6 | ~36 × x – 6 |
| Marble (average) | two.5 × 10 – 6 | 7.5 × x – six |
| Liquids | ||
| Ether | 1650 × ten – 6 | |
| Ethyl alcohol | 1100 × 10 – 6 | |
| Petrol | 950 × ten – 6 | |
| Glycerin | 500 × 10 – 6 | |
| Mercury | 180 × x – 6 | |
| Water | 210 × x – 6 | |
| Gases | ||
| Air and most other gases at atmospheric pressure | 3400 × 10 – 6 | |
Case 1. Calculating Linear Thermal Expansion: The Golden Gate Span
The principal span of San Francisco's Golden Gate Bridge is 1275 one thousand long at its coldest. The bridge is exposed to temperatures ranging from –15ºC to 40ºC. What is its change in length between these temperatures? Presume that the span is made entirely of steel.
Strategy
Utilize the equation for linear thermal expansion ΔL =α LΔT to summate the change in length , ΔL. Use the coefficient of linear expansion, α, for steel from Tabular array 1, and note that the change in temperature, ΔT, is 55ºC.
Solution
Plug all of the known values into the equation to solve for ΔL.
[latex]\Delta{L}=\alpha{L}\Delta{L}=\left(\frac{12\times10^{-6}}{^{\circ}\text{C}}\right)\left(1275\text{ m}\right)\left(55^{\circ}\text{C}\correct)=0.84\text{ 1000}\\[/latex]
Discussion
Although not large compared with the length of the span, this change in length is observable. It is generally spread over many expansion joints so that the expansion at each joint is small.
Thermal Expansion in Two and Three Dimensions
Objects aggrandize in all dimensions, every bit illustrated in Figure 2. That is, their areas and volumes, too as their lengths, increment with temperature. Holes also get larger with temperature. If y'all cut a pigsty in a metal plate, the remaining material will expand exactly as it would if the plug was notwithstanding in place. The plug would become bigger, and and then the hole must get bigger as well. (Call up of the ring of neighboring atoms or molecules on the wall of the hole every bit pushing each other farther apart as temperature increases. Obviously, the ring of neighbors must get slightly larger, so the hole gets slightly larger).
Thermal Expansion in Two Dimensions
For small temperature changes, the change in area ΔA is given by ΔA= 2αAΔT, where ΔA is the alter in surface area A, ΔT is the modify in temperature, and α is the coefficient of linear expansion, which varies slightly with temperature.
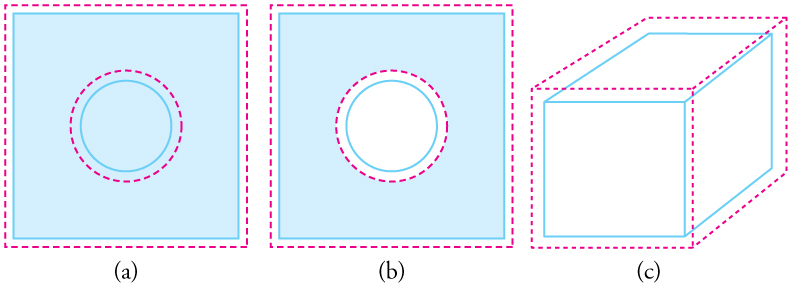
Effigy 2. In general, objects expand in all directions every bit temperature increases. In these drawings, the original boundaries of the objects are shown with solid lines, and the expanded boundaries with dashed lines. (a) Area increases considering both length and width increment. The expanse of a round plug also increases. (b) If the plug is removed, the hole it leaves becomes larger with increasing temperature, but equally if the expanding plug were still in place. (c) Volume likewise increases, because all three dimensions increase.
Thermal Expansion in Three Dimensions
The modify in volume ΔV is very almost ΔV = 3α FiveΔT. This equation is usually written as ΔV=βVΔT, where β is the coefficient of volume expansion and β≈ 3α. Note that the values of β in Table one are nigh exactly equal to 3α.
In full general, objects will expand with increasing temperature. Water is the most important exception to this rule. Water expands with increasing temperature (its density decreases) when it is at temperatures greater than 4ºC (40ºF). However, information technology expands with decreasing temperature when it is between +4ºC and 0ºC (40ºF to 32ºF). Water is densest at +4ºC. (Encounter Effigy 3.) Perchance the most striking effect of this miracle is the freezing of water in a swimming. When water near the surface cools down to 4ºC it is denser than the remaining water and thus will sink to the lesser. This "turnover" results in a layer of warmer h2o most the surface, which is then cooled. Eventually the pond has a uniform temperature of 4ºC. If the temperature in the surface layer drops below 4ºC, the water is less dense than the h2o below, and thus stays most the top. Every bit a result, the swimming surface can completely freeze over. The ice on acme of liquid water provides an insulating layer from winter'south harsh exterior air temperatures. Fish and other aquatic life tin survive in 4ºC h2o beneath water ice, due to this unusual characteristic of h2o. It also produces circulation of h2o in the pond that is necessary for a healthy ecosystem of the body of h2o.

Figure 3. The density of water as a function of temperature. Note that the thermal expansion is actually very small. The maximum density at +4ºC is but 0.0075% greater than the density at 2ºC, and 0.012% greater than that at 0ºC.
Making Connections: Existent-World Connections—Filling the Tank

Figure 4. Because the gas expands more the gas tank with increasing temperature, you tin't drive every bit many miles on "empty" in the summer as you can in the winter. (credit: Hector Alejandro, Flickr)
Differences in the thermal expansion of materials can atomic number 82 to interesting furnishings at the gas station. 1 example is the dripping of gasoline from a freshly filled tank on a hot day. Gasoline starts out at the temperature of the basis under the gas station, which is libation than the air temperature to a higher place. The gasoline cools the steel tank when it is filled. Both gasoline and steel tank expand as they warm to air temperature, but gasoline expands much more than steel, and so information technology may overflow.
This difference in expansion can as well cause problems when interpreting the gasoline gauge. The bodily corporeality (mass) of gasoline left in the tank when the gauge hits "empty" is a lot less in the summer than in the winter. The gasoline has the same book equally information technology does in the winter when the "add fuel" light goes on, but because the gasoline has expanded, at that place is less mass. If you are used to getting another forty miles on "empty" in the winter, beware—you will probably run out much more rapidly in the summer.
Case 2. Calculating Thermal Expansion: Gas vs. Gas Tank
Suppose your 60.0-L (fifteen.ix-gal) steel gasoline tank is total of gas, so both the tank and the gasoline have a temperature of 15.0ºC. How much gasoline has spilled past the time they warm to 35.0ºC?
Strategy
The tank and gasoline increase in volume, just the gasoline increases more, so the corporeality spilled is the difference in their volume changes. (The gasoline tank can be treated every bit solid steel.) We can utilize the equation for volume expansion to calculate the change in book of the gasoline and of the tank.
Solution
- Use the equation for volume expansion to summate the increment in volume of the steel tank: ΔV south =β s V sΔT.
- The increase in volume of the gasoline is given by this equation: Δ5 gas =β gas V gasΔT.
- Notice the departure in volume to determine the amount spilled asV spill=ΔV gas − ΔV south.
Alternatively, we tin can combine these three equations into a single equation. (Notation that the original volumes are equal.)
[latex]\begin{assortment}{lll}{V}_{\text{spill}}& =& \left({\beta }_{\text{gas}}-{\beta }_{\text{s}}\right)V\Delta T\\ & =& \left[\left(\text{950}-\text{35}\right)\times {\text{ten}}^{-6}/^{\circ}\text{C}\correct]\left(\text{lx}\text{.}0\text{L}\right)\left(\text{20}\text{.}0^{\circ}\text{C}\right)\\ & =& i\text{.}\text{x}\text{Fifty}\stop{array}\\[/latex]
Word
This amount is meaning, particularly for a 60.0-Fifty tank. The effect is and so striking because the gasoline and steel expand rapidly. The rate of change in thermal properties is discussed in the chapter Heat and Heat Transfer Methods.
If you effort to cap the tank tightly to prevent overflow, y'all will find that it leaks anyway, either around the cap or by bursting the tank. Tightly constricting the expanding gas is equivalent to compressing it, and both liquids and solids resist being compressed with extremely large forces. To avoid rupturing rigid containers, these containers have air gaps, which let them to expand and contract without stressing them.
Thermal Stress
Thermal stress is created past thermal expansion or contraction (run into Elasticity: Stress and Strain for a give-and-take of stress and strain). Thermal stress tin can exist destructive, such as when expanding gasoline ruptures a tank. It can also be useful, for case, when two parts are joined together by heating ane in manufacturing, and so slipping it over the other and assuasive the combination to absurd. Thermal stress can explicate many phenomena, such as the weathering of rocks and pavement by the expansion of ice when it freezes.
Instance iii. Calculating Thermal Stress: Gas Pressure level
What pressure would be created in the gasoline tank considered in Instance 2, if the gasoline increases in temperature from xv.0ºC to 35.0ºC without being allowed to expand? Assume that the bulk modulus B for gasoline is 1.00 × 109 N/m2.
Strategy
To solve this trouble, nosotros must apply the following equation, which relates a change in book ΔV to pressure:
[latex]\Delta{V}=\frac{ane}{B}\frac{F}{A}V_0\\[/latex]
where [latex]\frac{F}{A}\\[/latex] is pressure, V 0 is the original volume, and B is the majority modulus of the material involved. We will employ the amount spilled in Example 2 every bit the alter in volume, ΔV.
Solution
- Rearrange the equation for calculating pressure: [latex]P=\frac{F}{A}=\frac{\Delta{V}}{V_0}B\\[/latex].
- Insert the known values. The bulk modulus for gasoline is B = 1.00 × ten9 N/m2. In the previous example, the change in volume ΔV = i.ten L is the amount that would spill. Here, V 0 = 60.0 L is the original volume of the gasoline. Substituting these values into the equation, we obtain [latex]P=\frac{1.10\text{ L}}{60.0\text{ L}}\left(i.00\times10^9\text{ Pa}\right)=i.83\times10^7\text{ Pa}\\[/latex].
Word
This pressure is almost 2500 lb/in2, much more than a gasoline tank can handle.
Forces and pressures created by thermal stress are typically equally corking as that in the example to a higher place. Railroad tracks and roadways can buckle on hot days if they lack sufficient expansion joints. (Run into Effigy 5.) Power lines sag more than in the summertime than in the winter, and will snap in cold weather if there is insufficient slack. Cracks open and shut in plaster walls as a business firm warms and cools. Glass cooking pans will crevice if cooled quickly or unevenly, because of differential contraction and the stresses information technology creates. (Pyrex® is less susceptible because of its small coefficient of thermal expansion.) Nuclear reactor pressure vessels are threatened by overly rapid cooling, and although none have failed, several have been cooled faster than considered desirable. Biological cells are ruptured when foods are frozen, detracting from their taste. Repeated thawing and freezing accentuate the damage. Fifty-fifty the oceans can exist affected. A significant portion of the rise in sea level that is resulting from global warming is due to the thermal expansion of bounding main h2o.

Figure 5. Thermal stress contributes to the formation of potholes. (credit: Editor5807, Wikimedia Commons)
Metallic is regularly used in the man body for hip and knee joint implants. Most implants demand to be replaced over time because, amid other things, metal does not bond with os. Researchers are trying to find better metallic coatings that would allow metal-to-bone bonding. One challenge is to discover a coating that has an expansion coefficient similar to that of metal. If the expansion coefficients are too different, the thermal stresses during the manufacturing process lead to cracks at the coating-metal interface.
Another example of thermal stress is found in the oral cavity. Dental fillings can expand differently from tooth enamel. It tin give pain when eating ice cream or having a hot drinkable. Cracks might occur in the filling. Metal fillings (gold, silver, etc.) are being replaced past composite fillings (porcelain), which have smaller coefficients of expansion, and are closer to those of teeth.
Check Your Understanding
Two blocks, A and B, are fabricated of the same material. Block A has dimensions l×west×h=L× 250×50 and Block B has dimensions 2L× 2L× 2Fifty. If the temperature changes, what is
- the alter in the volume of the two blocks,
- the change in the cantankerous-sectional area l×west, and
- the change in the height h of the two blocks?

Effigy 6.
Solution
- The modify in volume is proportional to the original volume. Block A has a volume of Fifty× iiL×L= 2L 3.Block B has a volume of iiFifty× twoL× 2L= eightL iii , which is iv times that of Block A. Thus the change in book of Block B should be iv times the change in volume of Block A.
- The modify in expanse is proportional to the surface area. The cross-sectional area of Block A is L× 2L= 250 2 , while that of Block B is 2L× twoL= fourFifty 2. Because cantankerous-exclusive area of Cake B is twice that of Cake A, the change in the cantankerous-sectional expanse of Cake B is twice that of Cake A.
- The change in peak is proportional to the original top. Because the original superlative of Block B is twice that of A, the change in the height of Block B is twice that of Block A.
Section Summary
- Thermal expansion is the increment, or decrease, of the size (length, area, or volume) of a torso due to a modify in temperature.
- Thermal expansion is big for gases, and relatively small, but not negligible, for liquids and solids.
- Linear thermal expansion is Δ50= αFiftyΔT, where ΔL is the change in length Fifty, ΔT is the modify in temperature, and α is the coefficient of linear expansion, which varies slightly with temperature.
- The change in surface area due to thermal expansion is ΔA= 2αAΔT, where ΔA is the alter in area.
- The change in volume due to thermal expansion is ΔV= βVΔT, where β is the coefficient of volume expansion and β≈ 3α. Thermal stress is created when thermal expansion is constrained.
Conceptual Questions
- Thermal stresses caused by uneven cooling can easily pause glass cookware. Explain why Pyrex®, a glass with a small coefficient of linear expansion, is less susceptible.
- Water expands significantly when it freezes: a volume increment of nearly 9% occurs. As a result of this expansion and because of the germination and growth of crystals as water freezes, anywhere from 10% to thirty% of biological cells are outburst when animal or plant textile is frozen. Discuss the implications of this cell damage for the prospect of preserving human bodies past freezing so that they tin be thawed at some future date when it is hoped that all diseases are curable.
- One method of getting a tight fit, say of a metallic peg in a pigsty in a metal block, is to industry the peg slightly larger than the hole. The peg is and so inserted when at a different temperature than the block. Should the block exist hotter or colder than the peg during insertion? Explicate your answer.
- Does it really help to run hot water over a tight metallic hat on a glass jar before trying to open up it? Explain your reply.
- Liquids and solids expand with increasing temperature, considering the kinetic energy of a trunk's atoms and molecules increases. Explain why some materials shrink with increasing temperature.
Problems & Exercises
- The tiptop of the Washington Monument is measured to be 170 m on a day when the temperature is 35 . 0 º C . What will its height be on a day when the temperature falls to – 10 . 0 º C ? Although the monument is made of limestone, assume that its thermal coefficient of expansion is the same as marble's.
- How much taller does the Eiffel Belfry become at the end of a day when the temperature has increased by fifteen º C ? Its original height is 321 m and you can assume it is made of steel.
- What is the change in length of a 3.00-cm-long column of mercury if its temperature changes from 37 . 0 º C to xl . 0 º C , bold the mercury is unconstrained?
- How large an expansion gap should be left between steel railroad runway if they may reach a maximum temperature 35.0ºC greater than when they were laid? Their original length is 10.0 m.
- You are looking to buy a small slice of land in Hong Kong. The price is "only" $threescore,000 per foursquare meter! The land title says the dimensions are twenty m × 30 m. By how much would the full toll change if you lot measured the bundle with a steel tape measure on a 24-hour interval when the temperature was 20ºC higher up normal?
- Global warming will produce rise bounding main levels partly due to melting ice caps but likewise due to the expansion of h2o equally boilerplate ocean temperatures rise. To get some idea of the size of this effect, calculate the change in length of a column of water 1.00 km high for a temperature increase of one.00ºC. Note that this calculation is only gauge considering ocean warming is non uniform with depth.
- Show that 60.0 L of gasoline originally at 15.0ºC will expand to 61.one L when it warms to 35.0ºC, as claimed in Example 2.
- (a) Suppose a meter stick made of steel and one made of invar (an alloy of fe and nickel) are the same length at 0ºC. What is their divergence in length at 22.0ºC? (b) Repeat the calculation for ii 30.0-k-long surveyor's tapes.
- (a) If a 500-mL glass beaker is filled to the skirt with ethyl alcohol at a temperature of five.00ºC, how much will overflow when its temperature reaches 22.0ºC? (b) How much less water would overflow under the same conditions?
- Most automobiles have a coolant reservoir to catch radiator fluid that may overflow when the engine is hot. A radiator is fabricated of copper and is filled to its 16.0-L chapters when at 10.0ºC. What volume of radiator fluid will overflow when the radiator and fluid reach their 95.0ºC operating temperature, given that the fluid's volume coefficient of expansion is β= 400 × ten–6/ºC? Note that this coefficient is approximate, because most auto radiators have operating temperatures of greater than 95.0ºC.
- A physicist makes a cup of instant coffee and notices that, as the coffee cools, its level drops 3.00 mm in the glass cup. Prove that this subtract cannot be due to thermal contraction by computing the decrease in level if the 350cm3 of coffee is in a 7.00-cm-bore cup and decreases in temperature from 95.0ºC to 45.0ºC. (Most of the drop in level is really due to escaping bubbles of air.)
- (a) The density of water at 0ºC is very well-nigh 1000kg/m3 (information technology is actually 999.84 kg/m3), whereas the density of ice at 0ºC is 917 kg/grandiii. Calculate the pressure necessary to continue ice from expanding when it freezes, neglecting the outcome such a large pressure would have on the freezing temperature. (This problem gives you merely an indication of how large the forces associated with freezing water might be.) (b) What are the implications of this result for biological cells that are frozen?
- Show that β ≈ 3α, past computing the change in volume ΔFive of a cube with sides of length L.
Glossary
thermal expansion: the modify in size or volume of an object with change in temperature
coefficient of linear expansion: α, the change in length, per unit length, per 1ºC alter in temperature; a abiding used in the calculation of linear expansion; the coefficient of linear expansion depends on the material and to some degree on the temperature of the cloth
coefficient of book expansion:β, the change in volume, per unit of measurement book, per 1ºC modify in temperature
thermal stress: stress caused past thermal expansion or contraction
Selected Answers to Problems & Exercises
1. 169.98 chiliad
3. five.4 × 10−half dozen m
5. Because the area gets smaller, the price of the land DECREASES by ~$17,000.
7. [latex]\begin{array}{lll}V& =& {5}_{0}+\Delta 5={Five}_{0}\left(1+\beta \Delta T\right)\\ & =& \left(\text{60}\text{.}\text{00 L}\right)\left[i+\left(\text{950}\times {\text{10}}^{-6}/^{\circ}\text{C}\right)\left(\text{35}\text{.}0^{\circ}\text{C}-\text{15}\text{.}0^{\circ}\text{C}\right)\correct]\\ & =& \text{61}\text{.}1\text{L}\end{array}\\[/latex]
9. (a) 9.35 mL; (b) vii.56 mL
11. 0.832 mm
13. We know how the length changes with temperature: Δ50= α50 0ΔT. Also we know that the book of a cube is related to its length by V= L 3, so the last volume is then V= V 0+ Δ5= (L 0 + ΔL)3. Substituting for ΔL givesV= (Fifty 0 + αL 0ΔT)3= L0 3(1 + αΔT)3.
At present, because αΔT is minor, we tin can use the binomial expansion:V≈ 50 0 iii(ane + 3αΔT) = L 0 3+ 3αL 0 3ΔT.
And then writing the length terms in terms of volumes gives V= V 0+ ΔV≈ 5 0+ 3αV 0ΔT, and and then ΔV=βV 0ΔT≈ 3αV 0ΔT, or β≈ 3α.
Source: https://courses.lumenlearning.com/physics/chapter/13-2-thermal-expansion-of-solids-and-liquids/
0 Response to "Do All Liquids Expand at the Same Rate? How Do You Know?"
Postar um comentário